A normal male carrying a balanced translocation will produce both normal sperm cells and sperm cells in which the translocation gives rise to non-disjunction of the homolouge chromosomes which causes a production of sperm cells with deviating chromosome numbers.
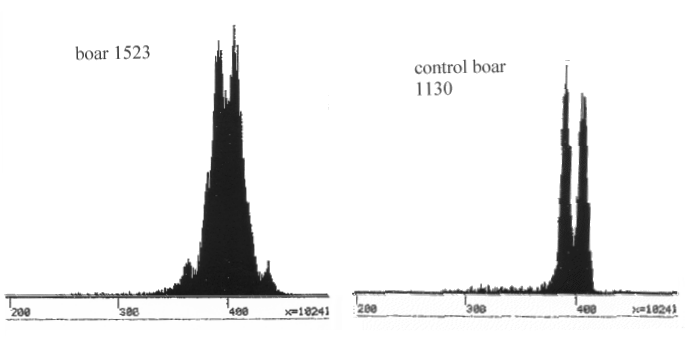
Figure 10.9 shows histograms of flow-cytometry of sperm cells from two boars. A histogram represents an intensity of fluorescence, which is proportional to the DNA-content in each sperm cell. Each histogram contains an intensity of around 3000 sperm cells. The boar to the right (Number 1130) is normal. It produces sperm cells with a Y- and an X-chromosome with a ratio of 1:1. A sperm cell containing a Y-chromosome has ca. 4 % less DNA than a sperm cell containing an X-chromosome. This is reflected in the fact that the mean value of two distributions differs with 4 % units.
The boar to the left (Number 1523) carries a translocation between chromosomes 1 and 17. When the sperm cells are formed in this boar, some errors occur with respect to those two chromosomes, which are involved in the translocation. Chromosome 1 is very large, representing around 9 % of the DNA-content of the sperm cell. Therefore, the histogram of this boar has two additional symmetrical tops, one on each side. The small top to the right corresponds to sperm cells with two chromosome 1's, while the top to the left corresponds to cells with no chromosome 1's. The two extra tops only appear when they are companied by an X- or a Y-chromosome, respectively. An additional chromosome 17 or the lack of one is not directly visible. This chromosome is a small one containing about 2 % of the DNA-content of a sperm cell. Therefore non-disjunction of this chromosome only adds to the general variation of the two main distributions. It is also clear that the translocation boar contains more variation than the normal one.
The investigations are published by Jensen, P.O. et al. 1993. Proceedings of 10th European Colloquium on Cytogenetics of Domestic Animals pp 104-108.
| Figure 10.9. Flow cytometry of boar sperm cells prestained with DAPI. The normal boar to the right has two tops, one for X- and another for Y-carrying cells. The boar with a translocation shows extra tops due to errors during the meiosis (non-disjunction). |
 |
Naturally the interest in utilizing flow-cytometry in a preparative mode is big. Which means production of sperm cells carrying either Y- or X-chromosomes. All species of mammals have some difference in the DNA-content in respectively Y- and X-carrying sperm cells (both equally large) from 4 to 5 %. Technically it is feasible to work with a coefficient of variations (CV) at around 1 %, which is the case in the example shown above of the normal boar.
Until year 2000 the results, with respect to getting live offspring after insemination with sorted sperm cells, have only been done on an experimental basis. The flow-cytometry cannot be done without the sperm cells have been treated with papain and stained with DAPI or other flourocromes. The papain treatment is needed for the staining of the DNA to be uniform.
Chromosome specific DNA-probes to identify non-disjunction in sperm cells
Another method of identifying non-disjunction chromosome errors is the utilization of strong chromosome specific probes. After in situ hybridisation of the probe onto slides with fixed sperm cells, the number of signals per cell can be counted. The possible outcome is sperm cells containing 0, 1 (normal) or 2 of the chromosomes in question, depending on the number of positive hybridising signals. Investigations of this type on humans give an average non-disjunction rate per chromosome in sperm cells of .2 to .3 %, which equals a total of 5 to 8 % non-disjunction per sperm cell. If the rate of error in the female is of the same magnitude, the absolute maximum fertility rate will be at around 85 %, since almost all non-balanced gametes would die in the first trimester of foetal life.
Development of similar chromosome specific probes for domestic animals are under way. The few results already obtained confirm the results derived from humans. Examples of utilization of mink chromosome specific probes on mink sperm cells can be seen here.